Cyclic voltammetry (CV) is a widely used electrochemical technique for analyzing the charge transfer of a redox-active species during a linearly cycled potential sweep. It provides valuable information about interfacial processes, redox thermodynamics, diffusion coefficients, electrode surface properties, and charge-transfer kinetics. However, despite its popularity, CV is often misunderstood due to the simultaneous occurrence of multiple processes, complicating the interpretation of voltammograms.
Let's consider the following redox reaction:
\[ Fe^{2+}\underset{k_b}{\overset{k_f}{\rightleftharpoons}}Fe^{3+}\]
The rate of this reaction can be described phenomenologically as:
\[ r=k_f[Fe^{2+}]-k_b[Fe^{3+}] \]
In the classical chemical kinetics, the kinetic constant depends exponentially with temperature according to the Arrhenius equation:
\[ k=A\exp\left(-\frac{Ea}{RT}\right) \]
This equation tells us that the rate constant increases exponentially with temperature, emphasizing the importance of thermal energy in overcoming the activation barrier. In electrochemistry the situation is slightly different. The rate constant for an electrochemical reaction depends on the applied potential (voltage), which provides the energy needed for the charge transfer reaction to occur at the electrode surface. This relationship is given by the Butler-Volmer equation, which describes the kinetics as a function of the difference between the applied potential and the equilibrium potential.
\[ k_f=k_0\exp\left(\frac{\alpha nF(E-E_0)}{RT}\right) \]
\[ k_b=k _0\exp\left(-\frac{(1-\alpha)nF(E-E_0)}{RT}\right )\]
In these equations, kf represents the rate constant for oxidation, while kb represents the rate constant for reduction. The overall charge transfer rate is governed by k0, the standard heterogeneous rate constant. F denotes the Faraday constant, and n is the number of electrons transferred. The symbol α represents the (dimensionless) transfer coefficient. E is the applied potential, and E0 is the formal redox potential—the potential at which the rates of oxidation and reduction are equal.
The figure below illustrates how the rates of oxidation and reduction change exponentially with the applied potential. When the polarization is more negative than E0, reduction is favoured. Conversely, when the polarization is more positive than E0, oxidation is favoured. However, it's important to note that both processes occur at all potentials. Changing the polarization favours one process over the other, but it doesn't mean that the other process ceases to exist.
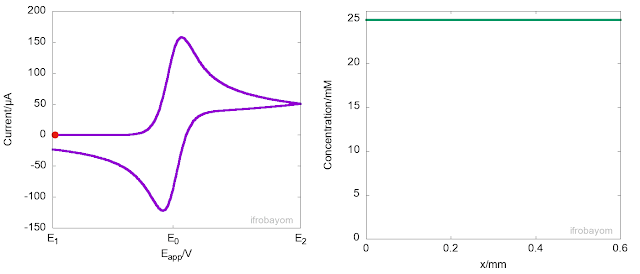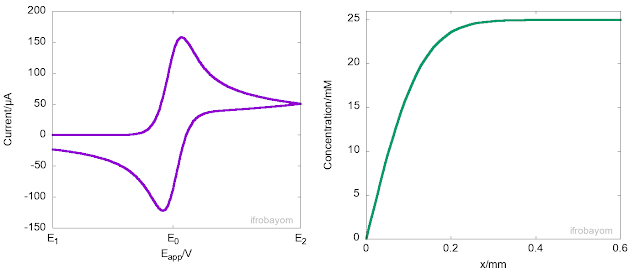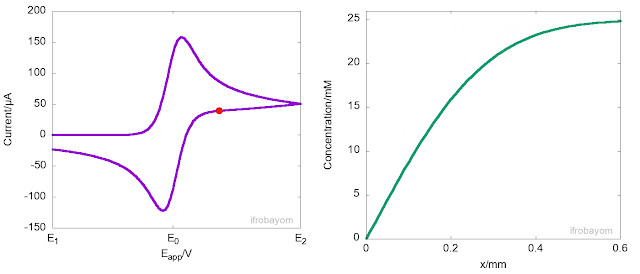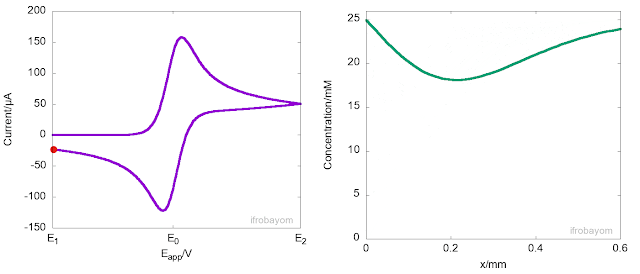
Another key aspect of these redox processes is that the charge transfer process only occurrs at the interface between the electrode and the electrolyte solution. When the applied potential is far from the equilibrium potential (E0), the rate of oxidation is very high, rapidly consuming all Fe2+ ions near the interface. As a result, a concentration gradient is generated, causing Fe2+ ions to diffuse from the bulk solution to the interface, leading to the formation of a diffusion layer. This diffusion layer has a concentration gradient that differs from the bulk solution. Typically, the length of this layer is about 400 micrometers. The following animation illustrates this behaviour.
As seen in the animation, the peak current is not an intrinsic property of the system but instead indicates the potential at which the rate of oxidation decreases due to a significant reduction in the concentration of Fe2+ near the interface. At this point, most of the Fe2+ ions available come from the diffusion from the bulk to the interface. However, because diffusion is a slow process, the current decays until it becomes null or reaches a steady state. It is often said, that at this stage, the current is controlled by mass transport.
According to Butler-Volmer equation, the reaction rate is proportional to the current, given by:
\[ i=FA(k_f[Fe^{2+}]_0 - k_b[Fe^{3+}]_0)\]
where A is the area of the electrode, and subscript 0 denotes the concentration of species near the electrode|electrolyte interface. However, as previously discussed, mass transport plays a crucial role in this process. The current can be defined as the flux of Fe2+ in the proximity of the electrode:
\[ i=FAD_{Fe^{2+}}\left(\frac{\partial [Fe^{2+}]}{\partial x}\right)_{x=0} \]
where DFe2+ is the diffusion coefficient of Fe2+. This equation emphasizes the importance of the mass transport in determining the current at the electrode surface.
Finally, it is very common to determine the half-wave potential (E1/2) which is the midpoint between the cathodic (Ec) and the anodic (Ea) potentials. This potential is usually associated to E0
\[ E_{1/2}=E_c - E_a \]
Voltammogram Simulator
The following app simulates a cyclic voltammogram and the corresponding diffusion layer, allowing the user to explore how different parameters affect the output. The parameters you can adjust include:
- [Re] and [Ox] (mM): Initial concentrations of the reduced and oxidised species.
- Diffusion coefficient of Re and Ox (DRe and DOx, in cm²·s⁻¹): Observe how diffusion affects the symmetry of the cyclic voltammogram (CV). Slider values are presented on a logarithmic scale (log10).
- Global electron transfer rate constant (k0, in cm·s⁻¹): Determines whether the redox process is reversible or irreversible. This parameter is also presented on a logarithmic scale.
- Bulk solution thickness (x, in mm): Represents the distance between the electrode and the edge of the bulk solution. Adjusting this parameter affects how strongly the diffusion layer influences the CV, particularly when x is comparable to the diffusion layer thickness.
- Charge transfer coefficient (α): Governs the symmetry of the electron transfer kinetics. Its influence is most significant at low k0 values but becomes negligible when k0 is large.
- Number of electrons transferred (n): This value can be fractional as it relates to the moles of charges transferred.
By adjusting these parameters, you can gain a deeper understanding of the factors influencing cyclic voltammetry and diffusion layers in electrochemical systems. In addition, the plot can be exported as csv files.
References
- Stephens, L.I., Mauzeroll, J. (2019). Demystifying Mathematical Modeling of Electrochemical Systems. Journal of Chemical Education, 96(10), 2217–2224. https://doi.org/10.1021/acs.jchemed.9b00542
- Compton, R.G., Laborda, E., Ward, K.R. (2014). Understanding Voltammetry: Simulation of Electrode Processes. Imperial College Press. https://doi.org/10.1142/p910
- Bard, A.J., Faulkner, L.R., White, H.S. (2022). Electrochemical Methods: Fundamentals and Applications (3rd ed.). John Wiley & Sons. https://doi.org/10.1023/A:1021637209564
- Elgrishi, N., Dempsey, J.L., et al. (2018). A Practical Beginner's Guide to Cyclic Voltammetry. Journal of Chemical Education, 95(2), 197–206. https://doi.org/10.1021/acs.jchemed.7b00361
If you want to cite this blog post, use:
Robayo, I. (2024). Understanding Cyclic Voltammetry: EC Mechanism [online].
Understanding cyclic voltammetry: Electron Transfer Mechanism.
Available at:
https://electrochemeisbasics.blogspot.com/2024/07/understanding-cyclic-voltammetry-ec.html
[Accessed Date Accessed].

Comments
Post a Comment