Exploring Cyclic Voltammetry: Unraveling the Dynamics of Chemical Reactions Coupled with Electron Transfer
In many cases, electrochemical systems are more complex than a simple interfacial electron transfer and may involve coupled chemical reactions. The presence of homogeneous chemical reactions in conjunction with the electrode process can significantly impact the electrochemical response of the system.
In this blog, we will explore the scenario where a homogeneous first-order reaction is followed by an interfacial electron transfer, as described by the following sequence reactions:
Here, k1 is the homogeneous rate constant, and the species BH is considered electro-inactive within the potential region under study. Additionally, we assume that the proton concentration is much higher than that of species B, allowing the homogeneous reaction to be approximated as a first-order reaction.
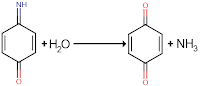
Examples of this type of mechanism include the oxidation of 1,4-aminophenol in protic media.
or the dissociation of a ligand from a metal complex:
In electrochemical literature, "C" typically denotes a chemical step, while "E" represents an electrochemical reaction. Therefore, the reaction sequence described above falls under what is known as the EC mechanism. When diffusion is the dominant mode of mass transport, the governing equations for this mechanism are:
In these equations, D represents the diffusion coefficient of the respective species, while k'1 is the apparent first-order rate constant defined as k'1 = [H+]k1. The rate of electron transfer at the interface is governed by the Butler–Volmer equation, which we discussed in detail in a previous post. Due to the coupled chemical reaction, the concentration of B decreases, leading to a reduced current during the reduction of B to produce A. The animation below illustrates how the cyclic voltammogram is influenced by an increase in pH while maintaining a constant value of k1.
When the rate of conversion of B to C is low, the behaviour of the EC mechanism closely resembles that of a stable, reversible electron transfer reaction. However, as the rate increases, the reduction peak begins to diminish because the chemical reaction depletes B. When the chemical reaction rate becomes significantly high, the electron transfer process transitions from being reversible to irreversible. Consequently, the oxidation peak shifts to more negative potentials and its height increases.
The following animation illustrates how the diffusion layer evolves when the pH decreases for species A and C. It clearly shows that at low pH levels, nearly all species A is converted to C. As a result, the concentration of A approaches zero, while C reaches the initial concentration of A.
A useful technique for studying this type of mechanism is spectroelectrochemistry. By coupling an appropriate spectroscopic method with electrochemistry, it becomes possible to detect species C, which cannot be observed using voltammetry alone.
CV Simulator
The following app simulates a cyclic voltammogram under the EC mechanism, providing insights into how different parameters influence the outcome. The app outputs both the voltammogram and the concentration profiles of species A and C, allowing you to explore various scenarios. The adjustable parameters include:
- Diffusion coefficient of A and B (DRe and DOx, respectively) in cm²/s: See how diffusion impacts the symmetry of the CV. It is assumed that the diffusion coefficient of C is equal to that of B. Note that slider values are on a logarithmic scale.
- The charge transfer coefficient (α): The influence of α is significant at low values of k0 but becomes negligible at high values of k0.
- The concentration of A ([Re]) in mM: Adjust the initial concentration of species A to observe its effect on the voltammogram.
- The global charge transfer constant (k0) in cm/s: Determine whether the redox process is reversible or irreversible. Like the diffusion coefficients, slider values for k0 are on a logarithmic scale.
- The length of the bulk solution (x) in mm: Explore how the CV changes when the bulk length becomes shorter than the diffusion layer.
- First-order kinetic constant (k1) in M⁻¹·s⁻¹: Control the rate at which B converts to C.
- The pH: Since the proton concentration is significantly higher than that of B, pH is treated as a parameter.
By adjusting these parameters, you can gain a deeper understanding of the factors that influence cyclic voltammetry and diffusion layers in electrochemical systems. In addition, the plots can be exported as CSV files.
References
- Compton, R.G., Laborda, E., Ward, K.R. (2014). Understanding Voltammetry: Simulation of Electrode Processes. Imperial College Press. https://doi.org/10.1142/p910
- Bard, A.J., Faulkner, L.R., White, H.S. (2022). Electrochemical Methods: Fundamentals and Applications (3rd ed.). John Wiley & Sons. https://doi.org/10.1023/A:1021637209564
- Britz, D., Strutwolf, J. (2016). Digital Simulation in Electrochemistry (4th ed.). Springer. https://doi.org/10.1007/978-3-319-30292-8
- Molina, A., Compton, R.G., et al. (2013). Effects of convergent diffusion and charge transfer kinetics on the diffusion layer thickness of spherical micro- and nanoelectrodes. Phys. Chem. Chem. Phys., 15, 7106–7113. https://doi.org/10.1039/C3CP50290B
- Elgrishi, N., Dempsey, J.L., et al. (2018). A Practical Beginner's Guide to Cyclic Voltammetry. J. Chem. Educ., 95(2), 197–206. https://doi.org/10.1021/acs.jchemed.7b00361
If you want to cite this blog post, use:
Robayo, I. (2024). Exploring Cyclic Voltammetry: Unraveling the Dynamics of Chemical Reactions Coupled with Electron Transfer. Available at:
https://electrochemeisbasics.blogspot.com/2024/08/exploring-cyclic-voltammetry-unraveling.html
[Accessed Date Accessed].



Comments
Post a Comment